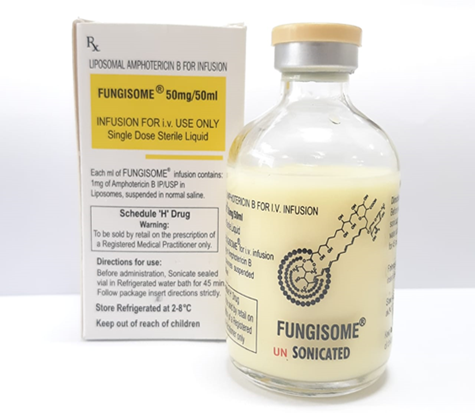
Fungisome
M/s Lifecare Innovations Private Limited, Lucknow, Uttar Pradesh
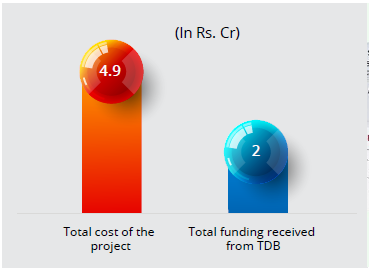
Technology Development Board (TDB) entered into an agreement with M/s Lifecare Innovations Private Limited for the project on “Development and Commercialization of Controlled Release Pharmaceuticals: Liposomal Formulation”. It received a loan assistance of Rs. 2 crore against the total project cost of Rs. 4.9 crore.
About the Technology
Liposomal Amphotericin B (LAmB-S) in Saline Suspension was a critical unmet medical need and a life-saving drug used for treatment of Kala-azar (Visceral Leishmaniasis; VL) and systemic fungal infections. Healthcare products developed using Liposomal Technology were entirely new to the world at that time. India was solely dependent on imported Liposomal Amphotericin B resulting in outgo of precious foreign exchange. FUNGISOME is both an import substitute and source of export income. FUNGISOME i.v. is the first in Asia and one of the only two Liposomal Amphotericin B injections in the world and is produced by M/s Lifecare Innovations Private Limited. It is the only hepato-safe and nephro-safe antifungal drug. The Nephrotoxicity, anti-fungal efficacy and daily dose of FUNGISOME are zero, >90%, 1-3mg/kg body wt./day in comparison to the imported LAmB which is 27%, 50% and 3-6mg/kg/day respectively, making FUNGISOME safer, more effective, and an economical drug to treat systemic fungal infections. FUNGISOME is effective in the treatment of MDR nosocomial infections viz. Candida auris, and Candida haemuloni that are resistant to imported LAmB. FUNGISOME has helped hospitals minimize mortality and morbidity caused by fungal infections and antifungal drugs. For VL, a single dose of FUNGISOME followed by oral Mitefosine for 14 days has given 100% treatment success, no relapse and no Post-kala-azar dermal leishmaniasis (PKDL) for at least 5 years, whereas, the imported LAmB failed in VL Clinical Trials in Africa and caused PKDL in 60% patients. PKDL is caused due to incomplete VL treatment because of which the parasite hibernates in skin and acts as a parasite reservoir for future VL episodes. FUNGISOME single dose treatment is a logistics boon for the VL treatment and PKDL prevention programs globally. FUNGISOME Gel is the only topical Amphotericin B for treatment of topical fungal infection of skin, burn victims, bed-sores etc. The gel is also the most effective treatment for Eye fungal infections. Liposomal Dithranol and Lipotar are novel formulations for treatment of Psoriasis that affect about 2% of the population. TDB funding helped to build the technology and healthcare products for medical needs.
Commercialisation of the Product
Lifecare Innovations Private Limited is a Biotechnology company pioneering in Liposomal Technology for unmet medical needs. This project was undertaken in Biotech Park, Lucknow. These healthcare products were manufactured using in-house technology. The products were commercialised on 11th May 2003. These healthcare medicines have outreach in more than 15 states in India. They also got international recognition including in North America, Europe, China, Japan, South Korea and Africa. Approximately 4,00,000 products have been sold till date.
Impact of funding
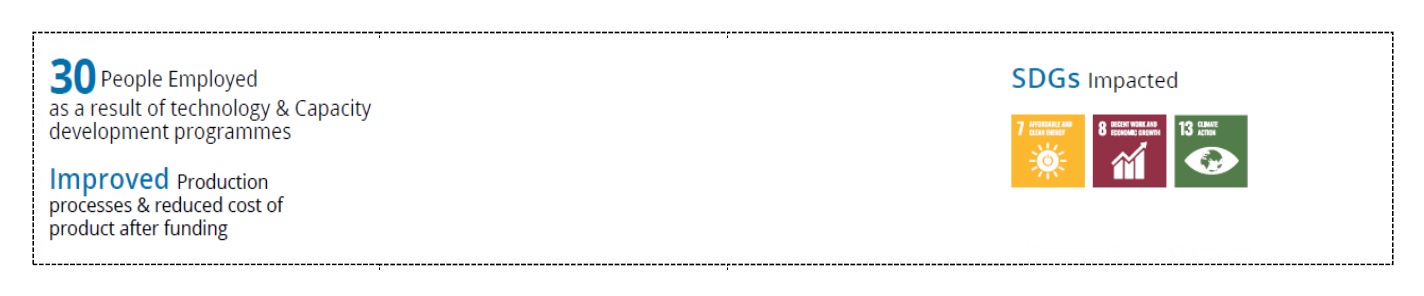
| Liposomal Amphotericin B in Saline Suspension for treatment of Kala-azar and Systemic Fungal infections, Liposomal Amphotericin B Gel for Topical and Ophthalmic Fungal Infections, Liposomal Dithranol for Psoriasis and Coaltar and Salysilic Acid- Phospholipid Structured Gel for Psoriasis F is sold not only in India but also in international markets including in North America, Europe, China, Japan, South Korea and Africa. |