Commercialization &setting up of manufacturing line for indigenous Medical LINAC (Siddharth-II - Medical Linear Accelerator)
M/s Panacea Medical Technologies Pvt Limited, Malur, Karnataka
Technology Development Board (TDB) has entered into an agreement with M/s Panacea Medical Technologies Pvt. Ltd. for the project on "Commercialization & setting up of manufacturing line for indigenous Medical LINAC". The project received the loan assistance of Rs. 12.5 crore against the total project cost of Rs. 45.3 crore. The application was received by TDB during F/Y 2019-20.
About the Technology

A medical linear accelerator (LINAC) machine is most commonly used for external beam radiation treatments for patients with cancer. It delivers high-energy x-rays or electrons to the region of the patient's tumor. The product is an improved version of existing products in the market. These treatments can be designed in such a way that they destroy the cancer cells while sparing the surrounding normal tissue. The LINAC is used to treat all body sites, using conventional techniques, Intensity Modulated Radiation Therapy (IMRT), Volumetric Modulated Arc Therapy (VMAT), Image Guided Radiation Therapy (IGRT), Stereotactic Radiosurgery (SRS) and Stereotactic Body Radiotherapy (SBRT). There is a high cost and limited access to Radiotherapy across several countries.
Commercialisation of the Product
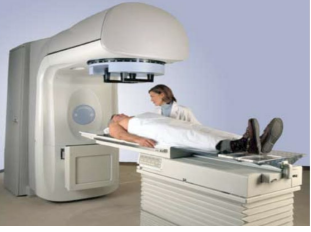
The project was undertaken in Karnataka. Panacea Medical Technologies Pvt. Ltd. was able to make products by adding more features in existing versions of the medical LINAC for radiotherapy. Today, Panacea’s 100+ radiotherapy machines are commercialized in South & South- East Asia and East & West Africa which have helped their hospitals to treat over 280,000 cancer patients. With installations in premier institutes across the globe, Panacea is leaving no stone unturned to ensure that every cancer patient has access to the best radiotherapy treatment.
Medical LINAC is a complex technology was received from SAMEER (Society for Applied Microwave Electronics Engineering & Research), an R&D Laboratory.
The company has developed the technology in house which is re cognized by DSIR. The company has worked on developing product and process features to be self-reliant and have established the product and processes to make the majority of the components in house by part count. The company has designed & built special purpose machines for production of critical components for indigenously manufacture. The product has received CE approval. TDB funding helped to build the technology. A total revenue of Rs. 8 crore has been generated in the year of commercialization and subsequent year itself.
Impact Of funding
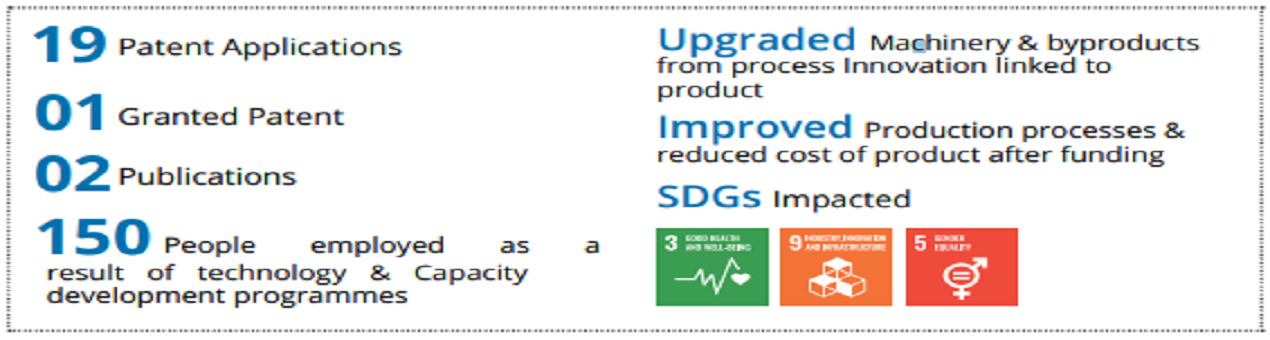
Medical LINAC for Radiotherapy by Panacea Medical Technologies Pvt. Ltd. is sold across India and has also received international recognition including in North America, Europe, South Korea, ASEAN, SAARC countries, and Africa.